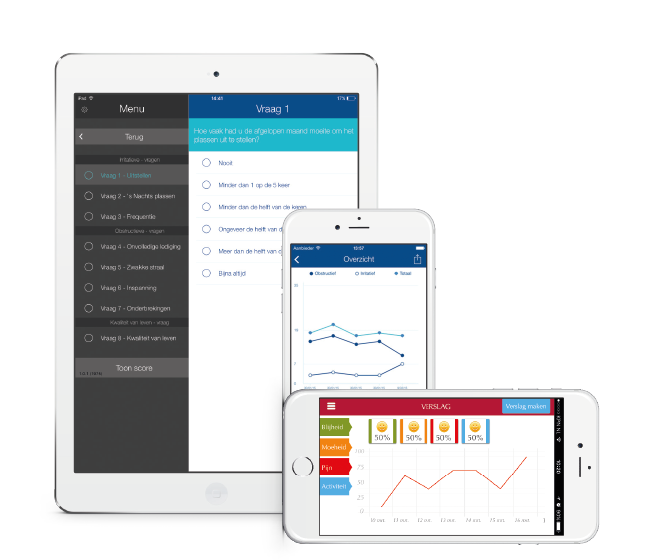
Pharmaceutical companies, manufacturers of medicinal products and medical devices are always on the lookout for opportunities to provide their target groups with added value. Several companies have recognized the increasing popularity of smartphones, tablets, and associated (medical) apps, and their potential to create this added value. But often the development of these apps is unknown territory.
Synappz’s methodology
The first step is to determine the medical issue at hand and for whom the app will be resolving a problem. This is essential if the objective is to create any kind of meaningful value for the end-user (as opposed to just a ‘marketing app’). End-users can be either healthcare professionals or, of course, patients. These end-users should ideally be involved in the development process.
After deciding upon the functionalities and the interaction design, via unique workshops, the app’s graphic design will be given shape. This design is often based on the company’s existing branding, products or services.
Once the clickable prototype has been approved, the encoding starts. If a database is being used, utmost care and consideration will be given to data security and privacy. It would be inadmissible for a pharmaceutical company to access the data if it pertains to patient data; cases such as these tend to be handled internally by the Regulatory Department. This department is closely involved in the process from the onset in order to ensure that all internal regulations are fully complied with. This is of particular importance when dealing with CE certification. Data can be hosted on our Cortex, a backend that has been especially created for medical applications.
Finally, when the app hits the Stores, a thorough marketing campaign will be carried out to properly launch it in the market. But, it doesn’t end there: we need to look at how popular the app is with the target group, measure its use, and think about future functionalities.
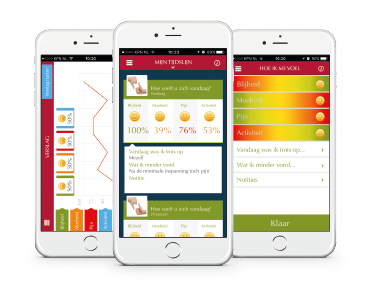
Medical apps for pharmaceutical companies
Medical apps can, depending on their functionalities, fall under CE regulations. This implies that a technical file needs to be written up during the app’s development stages. We always conduct a legal check if there is any uncertainty about whether or not the medical app falls under CE regulations. In addition, Synappz works with an internal Quality Management System that falls under the ISO9001 certification; this internal procedure ensures that the development and commercialization of medical apps is clearly detailed and monitored according to pre-established guidelines. This procedure can easily be taken over and handled by the Regulatory Department.

